This past Valentine’s Day, the Food and Drug Association approved a new method of birth control from a company called Agile Therapeutics, Inc. Although the product is not yet available to consumers, the company is hoping to begin the manufacturing process to formally introduce this new method by the end of the year. Known as Twirla, this contraceptive birth control patch is made for weekly use. Each patch delivers 120mcg of levonorgestrel and 30mcg of ethinyl estradiol through the skin and is made up of five layers in order to withstand daily use.
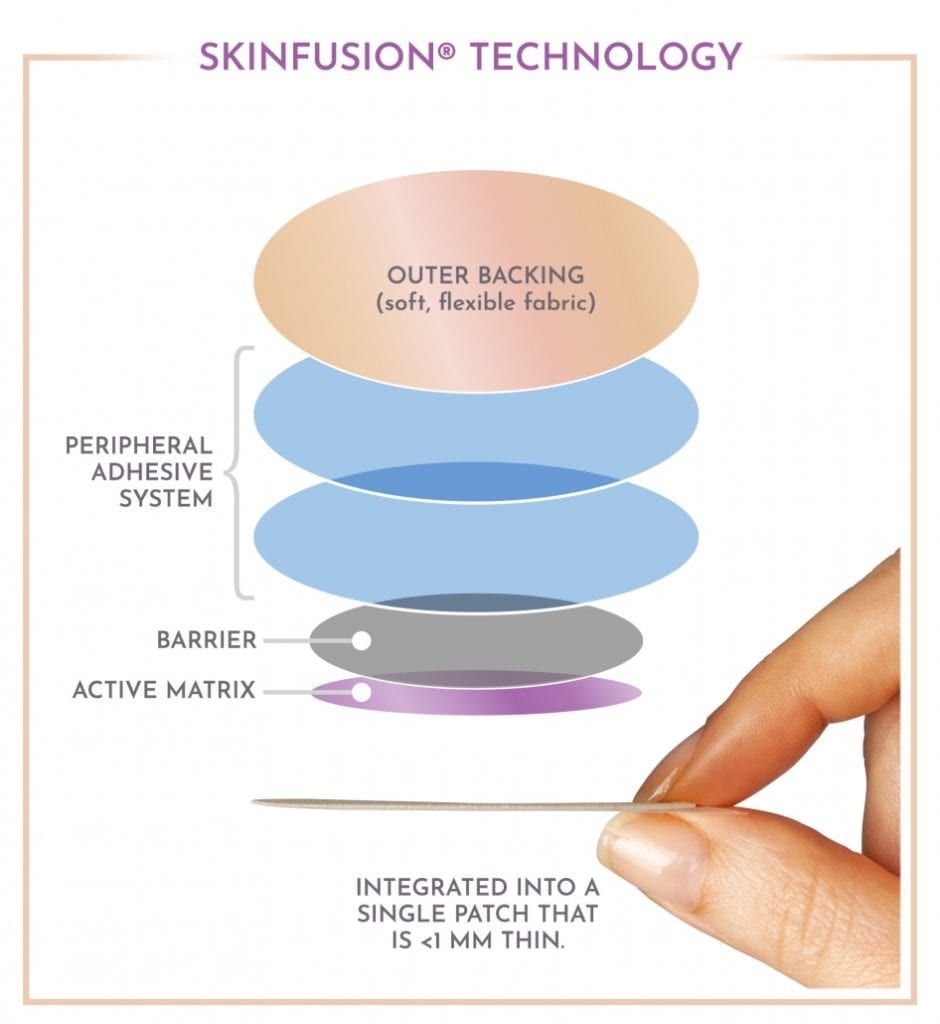
Each Twirla patch is applied once a week to the upper arm, buttocks, back, or lower abdomen for three weeks. After the third patch is removed, the fourth week is a patch-free week that allows for menstruation. One of the main benefits of this method is that it only requires women to remember their birth control about three times a month instead of daily. It is also considered to be less invasive than other birth control methods such as IUDs, implants, or the birth control shot.
Before Twirla, there was only one type of contraceptive birth control patch known as Xulane, which contains 150mcg of norelgestromin and 35 mcg of ethinyl estradiol. Xulane was introduced in 2014 to replace the Ortho Evra birth control patch when it was discontinued. Although Xulane is 99% effective against preventing pregnancy when used perfectly, it was found that women who used Xulane had higher blood serum concentrations of estrogen than women who used oral birth control. Unfortunately, these increased levels of estrogen increases the risk of blood clot formation in the legs and lungs.
The makers of Twirla worked hard to develop a low-dose hormonal contraceptive patch that offered the same effectiveness against preventing pregnancy. Since Twirla contains a lower dose of estrogen, the risk of blood clots in the legs and lungs is lower than other hormonal birth control methods with higher doses of estrogen. Like other forms of birth control, Twirla does not protect against sexually transmitted diseases or HIV.
Like other birth control methods, Twirla may not be right for everyone. For example, Twirla has been shown to be less effective at preventing pregnancy in women who weigh over 202 lbs or have a BMI greater than 30. However, this is also the case with Xulane, yet many women over 202 lbs still use it. Furthermore, Twirla is also not recommended for women who smoke, are at risk for thrombotic disease, experience migraines with aura, have liver disease, or have undiagnosed abnormal uterine bleeding.

Dr. Geoffrey Zann is a Certified Robotic Da Vinci Surgeon, Board-certified by the American College of Obstetricians and Gynecologists, and a Diplomat of the American Board Obstetrics of Gynecology. He has been a member of the American Society for Colposcopy and Cervical Pathology, American Association of Gynecologic Laparoscopists, and the Hugh R. K. Barber Obstetric and Gynecologic Society.
